The more general quality standard ISO 9001 serves as a major foundation for ISO 13485, the globally recognized quality management system (QMS) standard for the medical device sector. While maintaining quality and efficacy is their shared objective, ISO 13485 has important additions and revisions that are specifically designed to fulfill regulatory criteria pertaining to medical device performance and safety.
Section 1: Parallels In between the Standards
Both ISO 9001 and ISO 13485 use a similar basic framework and provide requirements for an all-encompassing Quality Management System (QMS).
| Foundations of Shared QMS | ISO 9001:2015 vs. ISO 13485:2016 |
| Process Approach | The foundation of both standards is a process approach to quality control. |
| Management Responsibilities | Both call for top management’s dedication to the QMS, which includes setting the quality targets and policy. |
| QMS Planning | In order to achieve quality goals, both require planning that considers the integrity of the QMS during the planning and execution of improvements. |
| Resource Management | Both need identifying and supplying resources, such as human resources (competence, training, and awareness) and infrastructure (e.g., facilities, process equipment). |
| Operation/Realization | Both contain customer-related procedures, design and development (Section 7.3 in 13485; Section 8.3 in 9001), and management of externally supplied goods and services (buying) are all covered in detail in both. |
| Improvement | Both emphasize measurement, analysis, and improvement processes, including mandatory requirements for implementing corrective action (CA) to prevent the recurrence of nonconformities. |
Part 2: Important Distinctions and ISO 13485 Details
A sector-specific standard called ISO 13485 was created for businesses engaged in one or more phases of a medical device’s life cycle. In contrast to ISO 9001, which places a strong emphasis on improving customer satisfaction and continual development, ISO 13485 places a higher priority on the legal standards necessary for performance and safety.
| ISO 13485 Specific Requirements | Key Focus | Corresponding ISO 9001 Clause Status |
| Regulatory Requirements | The organization must identify its role(s) under applicable regulatory requirements and incorporate these into the QMS. Compliance is the primary goal. | ISO 9001 focuses on statutory/regulatory compliance but lacks the specific emphasis on medical device safety regulations. |
| Risk Management | Requires the application of a risk-based approach to control appropriate QMS processes. The term “risk” specifically pertains to the safety or performance requirements of the medical device. | ISO 9001 applies general risk-based thinking to address risks and opportunities. |
| Documentation & Records | Requires the establishment and maintenance of one or more Medical Device Files for each device type or family, including general descriptions, specifications, manufacturing, packaging, and servicing procedures. Confidential health information protection is also required. | ISO 9001 requires documented information and records but has no equivalent clause for the Medical Device File. |
| Record Retention | Records must be retained for at least the lifetime of the medical device (as defined by the organization), but not less than two years from the device release. | ISO 9001 generally requires retaining documented information to support the operation of processes. |
| Outsourced Processes | Requires specific controls for outsourced processes, including written quality agreements, with controls proportionate to the risk involved. | ISO 9001 addresses external provision but does not explicitly require a written quality agreement. |
| Special Processes | Contains clauses with no equivalent in ISO 9001:2015, such as requirements for the cleanliness of product (7.5.2), installation activities (7.5.3), servicing activities (7.5.4), and particular requirements for sterile medical devices (7.5.5, 7.5.7). | These clauses contain requirements specific to the medical industry. |
| Post-Delivery Activities | Requires documented procedures for timely complaint handling (8.2.2), reporting adverse events/issuing advisory notices to regulatory authorities (8.2.3), and defining traceability for implantable medical devices (7.5.9.2). | ISO 9001 addresses customer feedback and post-delivery activities in a general sense. |
Part 3: Transitioning from ISO 9001 to ISO 13485
An organization currently certified to ISO 9001 has a substantial advantage, as the fundamental QMS framework (process approach, planning, resources, infrastructure) is already in place.
The process of moving from a general ISO 9001 QMS to the specialized ISO 13485 QMS primarily involves adapting and integrating the existing system to meet the rigorous, regulatory-driven requirements of the medical device sector.
Key areas for adaptation:
- Define Regulatory Context: The organization must first identify and document its specific role(s) in the medical device life-cycle (e.g., manufacturer, distributor, service provider) and determine all applicable regulatory requirements specific to its activities and markets.
- Integrate Risk Management (Safety Focus): The existing risk approach must be reframed to focus specifically on the safety and performance of the medical device, incorporating mandated risk management activities throughout the product realization process.
- Enhance Documentation: Create and maintain the sector-specific documentation, most critically the Medical Device File for each device type or family. Ensure all records meet the heightened retention requirements (lifetime of the device, minimum two years).
- Strengthen Control of Outsourcing: Implement written quality agreements with external suppliers for outsourced processes and ensure the level of control and monitoring is proportionate to the risk presented by the purchased product.
- Implement Specialized Process Controls: Document and implement procedures for activities specific to medical devices, such as:
- Validation of software used in the QMS and production.
- Requirements for cleanliness and contamination control.
- Procedures for installation and servicing (if applicable).
- Specific traceability requirements, particularly for implantable devices.
- Develop Post-Market Procedures: Establish robust, documented procedures for handling customer feedback and mandatory complaint handling, including mechanisms for evaluating the necessity of reporting adverse events and issuing advisory notices to regulatory authorities.
ISO 13485 requires establishing, implementing, and maintaining documentation for any procedure or activity required by the standard or applicable regulatory requirements. By using the existing ISO 9001 framework injection molding, the organization builds upon its foundation by adding the necessary regulatory rigor and documented controls required for medical device quality.

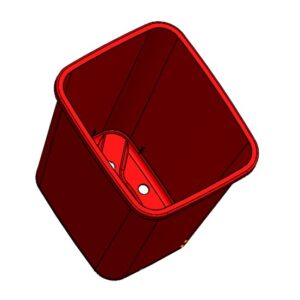
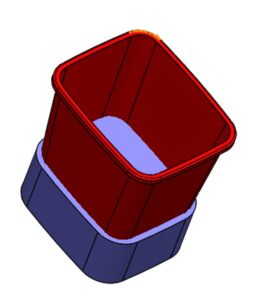

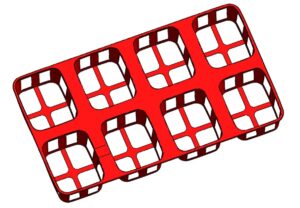 4. Add ribs using Rib tool with automatic draft.
4. Add ribs using Rib tool with automatic draft.